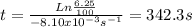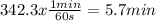Chemistry, 10.09.2019 22:30 liltinyhead
Acertain first-order reaction (a→products) has a rate constant of 8.10×10−3 s−1 at 45 ∘c. how many minutes does it take for the concentration of the reactant, [a], to drop to 6.25% of the original concentration? express your answer with the appropriate units.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3
You know the right answer?
Acertain first-order reaction (a→products) has a rate constant of 8.10×10−3 s−1 at 45 ∘c. how many m...
Questions


Biology, 05.01.2021 21:40

Mathematics, 05.01.2021 21:40



World Languages, 05.01.2021 21:40

History, 05.01.2021 21:40

Mathematics, 05.01.2021 21:40




Arts, 05.01.2021 21:40


Mathematics, 05.01.2021 21:40

English, 05.01.2021 21:40



Mathematics, 05.01.2021 21:40

English, 05.01.2021 21:40

Mathematics, 05.01.2021 21:40

![Ln [A] = -k.t + Ln [A]_{0}](/tpl/images/0227/1833/30b90.png) . Where [A] is the concentration of the reactant at any t time of the reaction,
. Where [A] is the concentration of the reactant at any t time of the reaction, ![[A]_{0}](/tpl/images/0227/1833/48818.png) is the concentration of the reactant at the beginning of the reaction and k is the rate constant.
is the concentration of the reactant at the beginning of the reaction and k is the rate constant. ![[A]=\frac{6.25}{100}.[A]_{0}](/tpl/images/0227/1833/4f27b.png) . And the rate constant (k) is 8.10×10−3 s−1
. And the rate constant (k) is 8.10×10−3 s−1![Ln \frac{6.25}{100}.[A]_{0} = -8.10x10^{-3}s^{-1}.t + Ln[A]_{0}](/tpl/images/0227/1833/587cc.png)
![Ln [A]_{0}.\frac{6.25}{100} - Ln [A]_{0} = -8.10x10^{-3}s^{-1}.t](/tpl/images/0227/1833/49854.png)
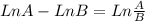
![Ln \frac{[A]_{0}}{[A]_{0}}.\frac{6.25}{100} = -8.10x10^{-3}s^{-1}.t](/tpl/images/0227/1833/72778.png)