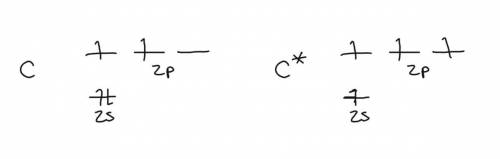Chemistry, 10.09.2019 21:30 sriharin58ozhj9m
(a) consider a carbon atom in its ground state. would such an atom offer a satisfactory model for the carbon of methane? if not, why not? (hint: consider whether a ground state carbon atom could be tetravalent, and consider the bond angles that would result if it were to combine with hydrogen atoms.)
(b) consider a carbon atom in the excited state. would such an atom offer a satisfactory model for the carbon of methane? if not, why not?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 23.06.2019 01:30
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1

You know the right answer?
(a) consider a carbon atom in its ground state. would such an atom offer a satisfactory model for th...
Questions

Social Studies, 20.09.2021 14:00



Mathematics, 20.09.2021 14:00







Mathematics, 20.09.2021 14:00




Mathematics, 20.09.2021 14:00


Mathematics, 20.09.2021 14:00