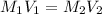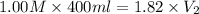Chemistry, 10.09.2019 21:30 hernandez48tur
Achemist must prepare 400 ml of 1.00m aqueous aluminum sulfate working solution. he'll do this by pouring out 1.82 mol/l aqueous aluminum sulfate stock solution into a graduated cylinder and diluting it with distilled water. how many ml of the aluminum sulfate stock solution should the chemist pour out?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3


You know the right answer?
Achemist must prepare 400 ml of 1.00m aqueous aluminum sulfate working solution. he'll do this by po...
Questions

English, 05.10.2021 21:30

History, 05.10.2021 21:30

Mathematics, 05.10.2021 21:30

Computers and Technology, 05.10.2021 21:30

Mathematics, 05.10.2021 21:30

Mathematics, 05.10.2021 21:30


SAT, 05.10.2021 21:30


Business, 05.10.2021 21:30


Mathematics, 05.10.2021 21:40

Social Studies, 05.10.2021 21:40

History, 05.10.2021 21:40

Mathematics, 05.10.2021 21:40





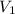 = 400 ml,
= 400 ml, 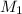 = 1.00 M
= 1.00 M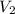 = ?,
= ?, 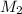 = 1.82 M
= 1.82 M