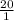Chemistry, 10.09.2019 20:30 dreyiamacc9695
The ph of blood depends on the [hco3-/h2co3] balance. ([h2co3] is equal to the amount of dissolved co2). calculate the bicarbonate (hco3-) : carbon dioxide ratio for a normal blood ph of 7.40. (the pka1 of carbonic acid is 6.10 at 37oc, body temperature). (a) 20 : 1 (b) 1.3 : 1 (c) 2 : 1 (d) 1 : 20 (e) 1 : 0.01

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3
You know the right answer?
The ph of blood depends on the [hco3-/h2co3] balance. ([h2co3] is equal to the amount of dissolved c...
Questions


Mathematics, 03.09.2020 22:01

Mathematics, 03.09.2020 22:01


History, 03.09.2020 22:01

Chemistry, 03.09.2020 22:01









History, 03.09.2020 22:01



Chemistry, 03.09.2020 22:01

Health, 03.09.2020 22:01

Mathematics, 03.09.2020 22:01

![[H_{2}CO_{3}]](/tpl/images/0226/9901/77e8d.png) =
= ![[CO_{2}]](/tpl/images/0226/9901/0a7e9.png)
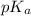 = 6.10
= 6.10![\frac{[HCO_^{-}{3}]}{[CO_{2}]}](/tpl/images/0226/9901/0b823.png) = ?
= ?![pK_{a} + log_{10} \frac{[Salt]}{[Acid]}](/tpl/images/0226/9901/394fa.png)
![pK_{a} + log_{10} \frac{[HCO^{-}_{3}]}{[H_{2}CO_{3}]}](/tpl/images/0226/9901/d9b18.png)
![log_{10} \frac{[HCO^{-}_{3}]}{[H_{2}CO_{3}]}](/tpl/images/0226/9901/e29e6.png)
![\frac{[HCO^{-}_{3}]}{[H_{2}CO_{3}]}](/tpl/images/0226/9901/4d8f1.png) = antilog (1.30)
= antilog (1.30) ![\frac{[HCO^{-}_{3}]}{[CO_{2}]}](/tpl/images/0226/9901/7a396.png) =
=