
Chemistry, 10.09.2019 18:30 kevinleon695
Which of the following is not a postulate of the kinetic molecular theory? select one: a. gas particles have most of their mass concentrated in the nucleus of the atom. b. the moving particles undergo perfectly elastic collisions with the walls of the container. c. the forces of attraction and repulsion between the particles are insignificant. d. the average kinetic energy of the particles is directly proportional to the absolute temperature. e. all of the above are postulates of the kinetic molecular theory.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 23.06.2019 08:00
If the solubility of a gas in water is 1.22 g/l at 2.75 atm, what is its solubility (in g/l) at 1.0 atm?
Answers: 1
You know the right answer?
Which of the following is not a postulate of the kinetic molecular theory? select one: a. gas part...
Questions




Mathematics, 02.12.2021 01:00






Computers and Technology, 02.12.2021 01:00

Mathematics, 02.12.2021 01:00

Mathematics, 02.12.2021 01:00

Mathematics, 02.12.2021 01:00


Chemistry, 02.12.2021 01:00


Mathematics, 02.12.2021 01:00

Computers and Technology, 02.12.2021 01:00

Mathematics, 02.12.2021 01:00

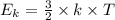 (where T is the absolute temperature and k the Boltzmann constant. This means that kinetic energy is directly proportional to temperature).
(where T is the absolute temperature and k the Boltzmann constant. This means that kinetic energy is directly proportional to temperature).


