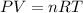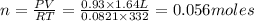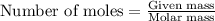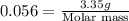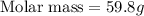Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1


Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1
You know the right answer?
A3.35 gram sample of an unknown gas is found to occupy a volume of 1.64 l at a pressure of 706 mmhg...
Questions

Mathematics, 14.09.2019 22:10


Mathematics, 14.09.2019 22:10

Mathematics, 14.09.2019 22:10


Biology, 14.09.2019 22:10



Mathematics, 14.09.2019 22:10

History, 14.09.2019 22:20



Mathematics, 14.09.2019 22:20






History, 14.09.2019 23:10