
Chemistry, 10.09.2019 00:30 bluetigerbird5323
Rate law equation the rate of a chemical reaction depends on the concentrations of the reactants. for the general reaction between a and b, aa+bb⇌cc+dd the dependence of the reaction rate on the concentration of each reactant is given by the equation called the rate law: rate=k[a]m[b]n where k is a proportionality constant called the rate constant. the exponent m determines the reaction order with respect to a, and n determines the reaction order with respect to b. the overall reaction order equals the sum of the exponents (m+n). what is the reaction order with respect to a?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3
You know the right answer?
Rate law equation the rate of a chemical reaction depends on the concentrations of the reactants. fo...
Questions

English, 30.08.2019 03:30

Mathematics, 30.08.2019 03:30

History, 30.08.2019 03:30



Spanish, 30.08.2019 03:30


English, 30.08.2019 03:30


Business, 30.08.2019 03:30


Chemistry, 30.08.2019 03:30

English, 30.08.2019 03:30




English, 30.08.2019 03:30



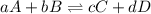
![Rate=k[A]^m[B]^n](/tpl/images/0226/2472/b2185.png)


