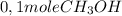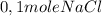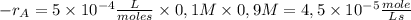Chemistry, 10.09.2019 00:20 disneyshree9427
The reaction described by the equation ch 3 cl + naoh → ch 3 oh + nacl follows the second-order rate law, rate = k [ ch 3 cl ] [ naoh ] . when this reaction is carried out with starting concentrations [ ch 3 cl ] = 0.2 m and [ naoh ] = 1.0 m , the measured rate is 1 × 10 − 4 mol l − 1 s − 1 . what is the rate after one-half of the ch 3 cl has been consumed? (caution: the initial concentrations of the starting materials are not identical in this experiment. hint: determine how much of the naoh has been consumed at this point and what its new concentration is, compared with its initial concentration.)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:50
It has been two weeks since charles met with daniel, a dietitian, who provided charles with a menu for weight loss. charles and his mother are going back to see daniel again with a chart of the food charles has eaten. the following lists what charles ate in one day: breakfast 1 banana, 1 cup of nonfat milk, 1 egg lunch 1 cup of carrots, 3 oz of steak, 1 apple, 1 cup of nonfat milk dinner 6 oz of skinless chicken, 1 baked potato, 3 oz of broccoli, 1 cup of nonfat milk
Answers: 1

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1


You know the right answer?
The reaction described by the equation ch 3 cl + naoh → ch 3 oh + nacl follows the second-order rate...
Questions



Mathematics, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00


Chemistry, 21.11.2020 01:00


Mathematics, 21.11.2020 01:00



History, 21.11.2020 01:00





History, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00

English, 21.11.2020 01:00

History, 21.11.2020 01:00

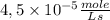
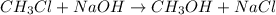
![-r_{A}=k \times [CH_{3}Cl] \times [NaOH]](/tpl/images/0226/2359/ac51f.png)
![1 \times 10^{-4}\frac{mole}{Ls}=k \times [0,2M] \times [1,0M] =5 \times 10^{-4}\frac{L}{mole s}](/tpl/images/0226/2359/a3f49.png)
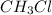 is consumed the mixture is composed by
is consumed the mixture is composed by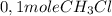 (half is consumed)
(half is consumed)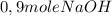 (by stoicheometry)
(by stoicheometry)