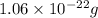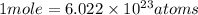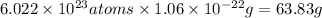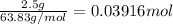Chemistry, 09.09.2019 21:30 ginocousins06
Acopper atom has a mass of 1 06 times 10^-22 g and a penny has a mass of 2.5 g. use this information to answer the questions below. be sure your answers have the correct number of significant digits. what is the mass of 1 mole of copper atoms? g how many moles of copper atoms have a mass equal to the mass of a penny?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

You know the right answer?
Acopper atom has a mass of 1 06 times 10^-22 g and a penny has a mass of 2.5 g. use this information...
Questions






Mathematics, 05.05.2020 23:15




English, 05.05.2020 23:15

History, 05.05.2020 23:15


History, 05.05.2020 23:15

English, 05.05.2020 23:15



Mathematics, 05.05.2020 23:15

Social Studies, 05.05.2020 23:15