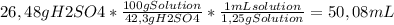Chemistry, 09.09.2019 19:10 runaway173
50 ml of a mixture consisting of 0.019 m cerium (iv) and 2.7 m h2so4, or sulfuric acid. begin by preparing 100 ml of 2.7 m h2so4 using the available solution of 42.3% w/w h2so4. this concentration unit, which may be less familiar to you, is a weight-to-weight percent (100.0 g of the solution contains 42.3 g of h2so4). the density of 42.3% w/w h2so4 is 1.25 g solution/ml solution. using a graduated cylinder, measure out the correct volume of 42.3% w/w h2so4 and slowly add it to a 100-ml volumetric flask that already contains approximately 25 ml of deionized water.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3


Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3
You know the right answer?
50 ml of a mixture consisting of 0.019 m cerium (iv) and 2.7 m h2so4, or sulfuric acid. begin by pre...
Questions



Geography, 21.09.2020 04:01


Mathematics, 21.09.2020 04:01


Mathematics, 21.09.2020 04:01


Health, 21.09.2020 04:01




Mathematics, 21.09.2020 04:01

Geography, 21.09.2020 04:01



Mathematics, 21.09.2020 04:01


Mathematics, 21.09.2020 04:01