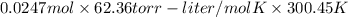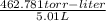Chemistry, 07.09.2019 05:30 dogsarecute278
The vapor pressure of a volatile liquid can be determined by slowly bubbling a known volume of gas through it at a known temperature and pressure. in an experiment, 5.01 l of n2 gas is passed through 7.9286 g of liquid benzene, c6h6, at 27.3 ∘c and atmospheric pressure. the liquid remaining after the experiment weighs 5.9987 g . assuming that the gas becomes saturated with benzene vapor and that the total gas volume and temperature remain constant, what is the vapor pressure of the benzene in torr?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1
You know the right answer?
The vapor pressure of a volatile liquid can be determined by slowly bubbling a known volume of gas t...
Questions


Mathematics, 23.04.2021 08:40

Biology, 23.04.2021 08:40


Mathematics, 23.04.2021 08:40

Biology, 23.04.2021 08:40

Mathematics, 23.04.2021 08:40

English, 23.04.2021 08:40

Biology, 23.04.2021 08:40


Mathematics, 23.04.2021 08:40

Mathematics, 23.04.2021 08:40



Mathematics, 23.04.2021 08:40




Mathematics, 23.04.2021 08:40

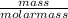
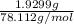
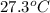 = 27.3 + 273.15 = 300.45 K
= 27.3 + 273.15 = 300.45 K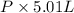 =
=