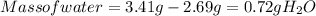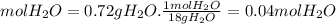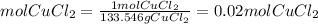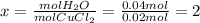Chemistry, 07.09.2019 04:30 batmannn1516
Ahydrate of copper (ii) chloride has the following formula: cucl2 - x h2o. the water in a 3.41-g sample of the hydrate was driven off by heating. the remaining sample had a mass of 2.69 g . find the number of waters of hydration (x) in the hydrate.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
Ahydrate of copper (ii) chloride has the following formula: cucl2 - x h2o. the water in a 3.41-g sa...
Questions

Geography, 03.02.2020 13:44

Mathematics, 03.02.2020 13:44

Mathematics, 03.02.2020 13:44

Mathematics, 03.02.2020 13:44

Mathematics, 03.02.2020 13:44





Chemistry, 03.02.2020 13:44



Mathematics, 03.02.2020 13:44




Mathematics, 03.02.2020 13:44


Computers and Technology, 03.02.2020 13:44

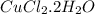
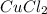 is the mass of remaining sample, because it is a product of loss of drying from initial sample. This means that the mass of water is the mass has been lost.
is the mass of remaining sample, because it is a product of loss of drying from initial sample. This means that the mass of water is the mass has been lost.