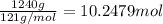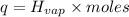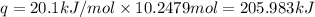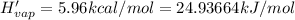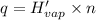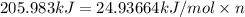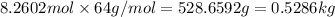Chemistry, 07.09.2019 01:30 krishimotam
Before the introduction of chlorofluorocarbons, sulfur dioxide (enthalpy of vaporization, 5.96 kcal/mol) was used in household refrigerators. what mass (in kg) of so2 must be evaporated to remove as much heat as evaporation of 1.24 kg of ccl2f2 (enthalpy of vaporization is 20.1 kj/mol)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

You know the right answer?
Before the introduction of chlorofluorocarbons, sulfur dioxide (enthalpy of vaporization, 5.96 kcal/...
Questions


Biology, 25.07.2021 05:50


Mathematics, 25.07.2021 05:50

Social Studies, 25.07.2021 05:50


Physics, 25.07.2021 05:50

Mathematics, 25.07.2021 05:50

English, 25.07.2021 05:50


Mathematics, 25.07.2021 05:50

English, 25.07.2021 05:50


Biology, 25.07.2021 05:50

Mathematics, 25.07.2021 05:50


Computers and Technology, 25.07.2021 05:50

Mathematics, 25.07.2021 05:50

Mathematics, 25.07.2021 05:50

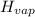 = 20.1 kJ/mol
= 20.1 kJ/mol