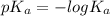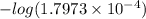(b) the conductivity of a 0.01 mol dm–3 solution of a monobasic organic acid in water is 5.07 × 10–2 s m–1. if the molar conductance at infinite dilution (λ°) of aqueous sodium chloride, sodium formate and hydrochloric acid are 1.264 × 10–2, 1.046 × 10–2 and 4.261 × 10–2 respectively at 25°c determine the acid dissociation constant and the pka for the acid.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1



Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1
You know the right answer?
(b) the conductivity of a 0.01 mol dm–3 solution of a monobasic organic acid in water is 5.07 × 10–2...
Questions



Mathematics, 02.02.2020 15:46

Biology, 02.02.2020 15:47

Mathematics, 02.02.2020 15:47




Mathematics, 02.02.2020 15:47

Mathematics, 02.02.2020 15:47

Biology, 02.02.2020 15:47

Mathematics, 02.02.2020 15:47

Computers and Technology, 02.02.2020 15:47





History, 02.02.2020 15:47

Biology, 02.02.2020 15:47

Mathematics, 02.02.2020 15:47

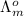 (NaCl) =
(NaCl) = 

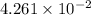
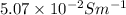
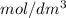
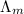 ) of monobasic acid is calculated as follows.
) of monobasic acid is calculated as follows.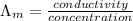
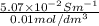
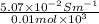
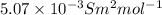
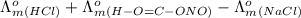
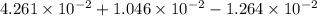
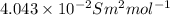
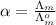
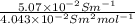
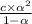
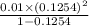
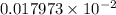
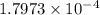
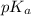 and
and 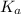 is as follows.
is as follows.