
Chemistry, 07.09.2019 01:10 jiedwards3835
For the reaction pc15 (8) pc13 (g) + cl2 (g) k = 0.0454 at 261 °c. if a vessel is filled with these gases such that the initial concentrations are [pc15) = 0.20 m, [pc13] = 0.20 m, and (cl21 = 2.5 m, in which direction will a reaction occur and why? a) toward products because qc = 0.56 b) toward reactants because qc = 2.5 c) toward products because qc = 2.8 d) toward reactants because qc = 0.0454 e) it is at equilibrium because qc = 1

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
You know the right answer?
For the reaction pc15 (8) pc13 (g) + cl2 (g) k = 0.0454 at 261 °c. if a vessel is filled with these...
Questions


Geography, 17.09.2019 11:00


Biology, 17.09.2019 11:00

Mathematics, 17.09.2019 11:00

Mathematics, 17.09.2019 11:00

Mathematics, 17.09.2019 11:00

Biology, 17.09.2019 11:00


Mathematics, 17.09.2019 11:00



Mathematics, 17.09.2019 11:00

History, 17.09.2019 11:00


Mathematics, 17.09.2019 11:00

Mathematics, 17.09.2019 11:00



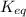 is samller than
is samller than 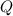 of the reaction . So,the reaction will shift towards the left i.e. towards the reactant side.
of the reaction . So,the reaction will shift towards the left i.e. towards the reactant side.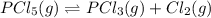
![Q=\frac{[PCl_3][Cl_2]}{[[PCl_5]^1}](/tpl/images/0224/9376/2eff0.png)
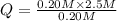
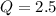
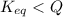 , the reaction will shift towards the left i.e. towards the reactant side.
, the reaction will shift towards the left i.e. towards the reactant side.

