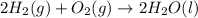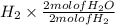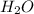Chemistry, 06.09.2019 23:30 quissowavyquis8484
Consider the balanced chemical equation that follows. you are asked to determine how many moles of water you can produce from 4.0 mol of hydrogen and excess oxygen. (excess oxygen means that so much oxygen is available it will not run out.) which of the numbers that appear in the balanced chemical equation below are used to perform this calculation?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1
You know the right answer?
Consider the balanced chemical equation that follows. you are asked to determine how many moles of w...
Questions

Health, 28.07.2019 19:00


Social Studies, 28.07.2019 19:00


Mathematics, 28.07.2019 19:00

Biology, 28.07.2019 19:00