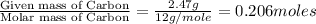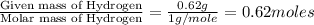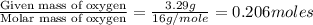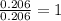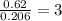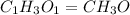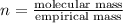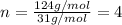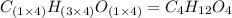Chemistry, 06.09.2019 23:20 zoewilliamss26
Ethylene glycol used in automobile antifreeze and in the production of polyester. the name glycol stems from the sweet taste of this poisonous compound. combustion of 6.38 g of this compound gives 9.06 g of co2 and 5.58 g of h2o. the compound only contains c, h, and o. if the molecular mass of this compound is 124 amu, what is the empirical and molecular formula?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2


Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
You know the right answer?
Ethylene glycol used in automobile antifreeze and in the production of polyester. the name glycol st...
Questions


English, 03.08.2019 12:00

Mathematics, 03.08.2019 12:00

Mathematics, 03.08.2019 12:00



Mathematics, 03.08.2019 12:00










History, 03.08.2019 12:00

History, 03.08.2019 12:00


History, 03.08.2019 12:00

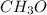 and
and 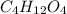
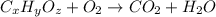
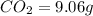
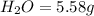
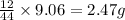 of carbon will be contained.
of carbon will be contained.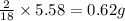 of hydrogen will be contained.
of hydrogen will be contained.