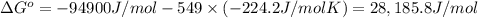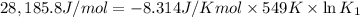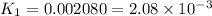Chemistry, 06.09.2019 23:20 zhellyyyyy
Determine the equilibrium constant for the following reaction at 549 k. ch2o(g) + 2 h2(g) → ch4(g) + h2o(g) δh° = -94.9 kj; δs°= -224.2 j/k determine the equilibrium constant for the following reaction at 549 k. ch2o(g) + 2 h2(g) → ch4(g) + h2o(g) δh° = -94.9 kj; δs°= -224.2 j/k 1.07 x 109 481 2.08 x 10-3 9.35 x 10-10 1.94 x 10-12

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 23.06.2019 11:30
Which of these have the same number of particles as 1 mole of water h2o
Answers: 1

Chemistry, 23.06.2019 11:30
The density of e85 fuel is 0.801 g/ml. what is the mass of 1.00 gallon of the fuel? (1 gal. = 3.785 l)
Answers: 3

Chemistry, 23.06.2019 12:30
How do you interpret a chromatogram for what mixtures contain?
Answers: 3
You know the right answer?
Determine the equilibrium constant for the following reaction at 549 k. ch2o(g) + 2 h2(g) → ch4(g) +...
Questions

Mathematics, 16.11.2020 15:10

Mathematics, 16.11.2020 15:10

Mathematics, 16.11.2020 15:10


Mathematics, 16.11.2020 15:10

Computers and Technology, 16.11.2020 15:20


Mathematics, 16.11.2020 15:20

English, 16.11.2020 15:20

Business, 16.11.2020 15:20


Physics, 16.11.2020 15:20

Mathematics, 16.11.2020 15:20

Social Studies, 16.11.2020 15:20



English, 16.11.2020 15:20

English, 16.11.2020 15:20


Mathematics, 16.11.2020 15:20

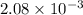 .
.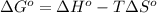
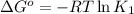
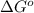 = Gibbs free energy
= Gibbs free energy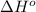 = Enthalpy of reaction
= Enthalpy of reaction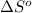 = Entropy of reaction
= Entropy of reaction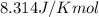
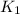 = equilibrium constant at T
= equilibrium constant at T