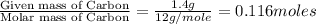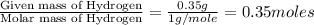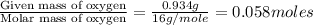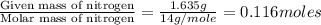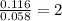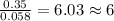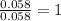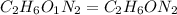Dimethyl nitrosamine is a known carcinogen. it can be formed in the intestinal tract when digestive juices react with the nitrite ion is preserved and smoked meats. it is made up of carbon, hydrogen, nitrogen, and oxygen atoms. a 4.319 g sample of dimethyl nitrosamine burned in oxygen yields 5.134 g of co2 and 3.173 g of h2 o. the compound contains 37,82% by mass of nitrogen. what is the empirical formula of dimethyl nitrosamine?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
True or false, the three major scales used to measure earthquakes are mercalli scale, richter scale and magnitude scale
Answers: 2

Chemistry, 22.06.2019 00:30
Sarah wants to know where in her garden chamomile would grow the best. she thinks chamomile will grow best in the corner of the garden that gets the most sunlight. to test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. which of the following variables will sarah need to measure to know which group of plants grew best? a. the location of the plants b. the type of plants c. the height of the plants d. the amount of water she gives the plants
Answers: 1

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2
You know the right answer?
Dimethyl nitrosamine is a known carcinogen. it can be formed in the intestinal tract when digestive...
Questions

Mathematics, 22.03.2022 17:50


Mathematics, 22.03.2022 18:00




Mathematics, 22.03.2022 18:10




Chemistry, 22.03.2022 18:40

Mathematics, 22.03.2022 18:40


Mathematics, 22.03.2022 18:50

Business, 22.03.2022 18:50




Mathematics, 22.03.2022 18:50

Mathematics, 22.03.2022 19:00

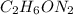
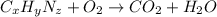
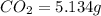
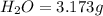
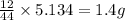 of carbon will be contained.
of carbon will be contained.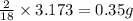 of hydrogen will be contained.
of hydrogen will be contained.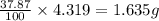 of nitrogen
of nitrogen