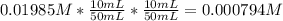Chemistry, 06.09.2019 18:30 angelteddy033
The molar concentration (m) of a solution prepared by dissolving 0.2362g of cr(no3)3 in a 50-ml volumetric flask is 0.01985m, where the molecular weight for cr(no3)3 = 238.01g/mol.
a. suppose you want to prepare another solution containing chromium nitrate that is 25 times less concentrated than the one prepared above. given a choice of 10-ml and 5-ml pipets and 50-ml and 100-ml volumetric flasks, explain how you would proceed in preparing the new diluted solution. in addition, calculate the concentration for the new diluted solution. show all work. your final value should have the correct unit and number of significant figures. hint: you will most likely need two dilution steps in order to obtain the desired concentration. note: you may not reuse the same pipet or combine different pipets within the same dilution step. you may reuse the pipet and/or volumetric flask in the different dilution step.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1


Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3
You know the right answer?
The molar concentration (m) of a solution prepared by dissolving 0.2362g of cr(no3)3 in a 50-ml volu...
Questions

Health, 21.12.2020 14:00


Physics, 21.12.2020 14:00

Chemistry, 21.12.2020 14:00

Mathematics, 21.12.2020 14:00



Mathematics, 21.12.2020 14:00

Computers and Technology, 21.12.2020 14:00

Chemistry, 21.12.2020 14:00

Mathematics, 21.12.2020 14:00

Mathematics, 21.12.2020 14:00

Business, 21.12.2020 14:00

Engineering, 21.12.2020 14:00



Mathematics, 21.12.2020 14:00

Social Studies, 21.12.2020 14:00