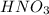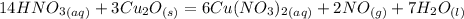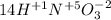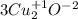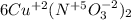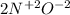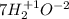Chemistry, 06.09.2019 17:20 mercedespennewp72wea
What is the oxidizing agent in the following reaction? 14 hno3(aq) + 3 cu20(s) = 6 cu(no3)2(aq) + 2 no(g) + 7 h2o(l) hno3 cu20 cu(no3)2 o ld no

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1


Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1
You know the right answer?
What is the oxidizing agent in the following reaction? 14 hno3(aq) + 3 cu20(s) = 6 cu(no3)2(aq) + 2...
Questions


History, 25.06.2019 21:00

Social Studies, 25.06.2019 21:00




History, 25.06.2019 21:00

Mathematics, 25.06.2019 21:00


History, 25.06.2019 21:00

Biology, 25.06.2019 21:00

Mathematics, 25.06.2019 21:00

Mathematics, 25.06.2019 21:00



Mathematics, 25.06.2019 21:00

Mathematics, 25.06.2019 21:00


History, 25.06.2019 21:00

Health, 25.06.2019 21:00