
Chemistry, 06.09.2019 17:20 jerenasmith8
17) chlorine and fluorine react to form gaseous chlorine trifluoride. you start with 1.75 mole of chlorine and 3.68 moles of fluorine. a. write the balanced equation for the reaction. b. what is the limiting reactant? c. find the moles of clf3produced? d. find the moles of excess left over

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
17) chlorine and fluorine react to form gaseous chlorine trifluoride. you start with 1.75 mole of ch...
Questions

Mathematics, 10.02.2021 23:10




Mathematics, 10.02.2021 23:10





Mathematics, 10.02.2021 23:10

History, 10.02.2021 23:10

Mathematics, 10.02.2021 23:10





English, 10.02.2021 23:10



Mathematics, 10.02.2021 23:10

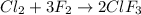
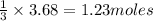 of chlorine
of chlorine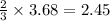 moles of chlorine trifluoride.
moles of chlorine trifluoride.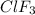 are produced.
are produced.

