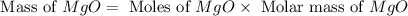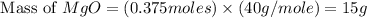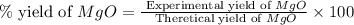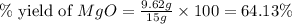Magnesium oxide can be made by heating magnesium metal in the presence of the oxygen. the balanced equation for the reaction is 2 mg(s) + o2(g) → 2 mgo(s) now consider that you react 10.0 g mg with 6.00 g o2 gas. if you were able to collect 9.62 g of mgo, what would be your percent yield for the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1
You know the right answer?
Magnesium oxide can be made by heating magnesium metal in the presence of the oxygen. the balanced e...
Questions

Mathematics, 07.12.2020 20:50


Biology, 07.12.2020 20:50


Mathematics, 07.12.2020 20:50






Mathematics, 07.12.2020 20:50


Mathematics, 07.12.2020 20:50

Mathematics, 07.12.2020 20:50

Computers and Technology, 07.12.2020 20:50

History, 07.12.2020 20:50

Mathematics, 07.12.2020 20:50


Social Studies, 07.12.2020 20:50

Mathematics, 07.12.2020 20:50

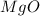 is, 64.13 %
is, 64.13 %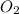 = 6 g
= 6 g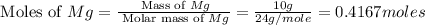
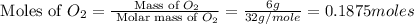
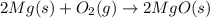
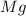
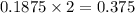 moles of
moles of