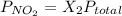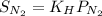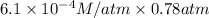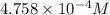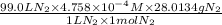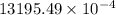Chemistry, 06.09.2019 16:20 cindyc1103
Calculate the mass of nitrogen dissolved at room temperature in an 99.0 l home aquarium. assume a total pressure of 1.0 atm and a mole fraction for nitrogen of 0.78. express your answer using two significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2


Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1
You know the right answer?
Calculate the mass of nitrogen dissolved at room temperature in an 99.0 l home aquarium. assume a to...
Questions


Physics, 06.01.2021 03:00


Mathematics, 06.01.2021 03:00



History, 06.01.2021 03:00

Advanced Placement (AP), 06.01.2021 03:00


Physics, 06.01.2021 03:00






Health, 06.01.2021 03:00

Biology, 06.01.2021 03:00

Health, 06.01.2021 03:00


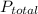 = 1 atm,
= 1 atm, 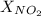 = 0.78
= 0.78