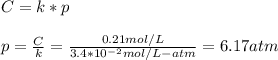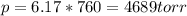Chemistry, 05.09.2019 23:30 avalianagames
Part a an unopened can of soda has an aqueous co2 concentration of 0.21 m at 25.0 °c. what is the partial pressure of the gas in the can in torr? the henry's law constant for co2 at 25 °c is 3.4 × 10−2 mol/l-atm.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
Part a an unopened can of soda has an aqueous co2 concentration of 0.21 m at 25.0 °c. what is the pa...
Questions

Mathematics, 19.07.2019 10:30

Mathematics, 19.07.2019 10:30





History, 19.07.2019 10:30


Physics, 19.07.2019 10:30




History, 19.07.2019 10:30

Social Studies, 19.07.2019 10:30

Mathematics, 19.07.2019 10:30


Mathematics, 19.07.2019 10:30



History, 19.07.2019 10:30