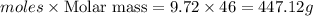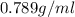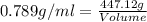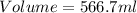Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
Near room temperature the density ofwater is 1 g/ml and the densityof ethanol is 0.789 g/ml . what v...
Questions

English, 16.11.2019 04:31

Mathematics, 16.11.2019 04:31

English, 16.11.2019 04:31


Mathematics, 16.11.2019 04:31

Advanced Placement (AP), 16.11.2019 04:31



Mathematics, 16.11.2019 04:31



Mathematics, 16.11.2019 04:31


Mathematics, 16.11.2019 04:31

Mathematics, 16.11.2019 04:31

Engineering, 16.11.2019 04:31

Chemistry, 16.11.2019 04:31



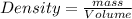
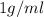
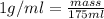
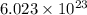 of particles.
of particles.