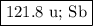Chemistry, 05.09.2019 22:30 shayambros
An element has two naturally occurring isotopes. isotope 1 has a mass of 120.9038 amu and a relative abundance of 57.4%, and isotope 2 has a mass of 122.9042 amu. find the atomic mass of this element and identify it.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3
You know the right answer?
An element has two naturally occurring isotopes. isotope 1 has a mass of 120.9038 amu and a relative...
Questions



Mathematics, 01.04.2020 19:14

Biology, 01.04.2020 19:14


Mathematics, 01.04.2020 19:14






Mathematics, 01.04.2020 19:14



Computers and Technology, 01.04.2020 19:14

Mathematics, 01.04.2020 19:14


Computers and Technology, 01.04.2020 19:14