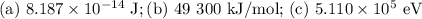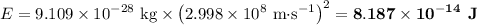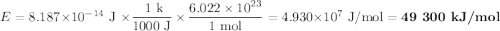Consider a process in which two gamma-ray photons are released when an electron and a positron are annihilated. [note that the mass of a positron is equal to that of an electron, and the gamma-ray photon has no mass.]
(a) what is the final energy of each photon (in j units)?
(b) what is the final energy of each photon (in kj/mol units)?
(c) what is the final energy of each photon (in ev units)?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2
You know the right answer?
Consider a process in which two gamma-ray photons are released when an electron and a positron are a...
Questions

Business, 15.09.2021 22:20



Geography, 15.09.2021 22:20


Geography, 15.09.2021 22:20

Physics, 15.09.2021 22:20





Chemistry, 15.09.2021 22:20


Mathematics, 15.09.2021 22:20


Chemistry, 15.09.2021 22:20


Mathematics, 15.09.2021 22:20

Chemistry, 15.09.2021 22:20

Medicine, 15.09.2021 22:20