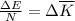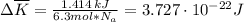Chemistry, 05.09.2019 21:20 jackchelly
The temperature of 6.30 mol of an ideal monatomic gas is raised 18.0 k in an adiabatic process. what are (a) the work w done by the gas, (b) the energy transferred as heat q, (c) the change δeint in internal energy of the gas, and (d) the change δk in the average kinetic energy per atom?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
The temperature of 6.30 mol of an ideal monatomic gas is raised 18.0 k in an adiabatic process. what...
Questions


Mathematics, 30.03.2020 21:46

History, 30.03.2020 21:46



Mathematics, 30.03.2020 21:46

Mathematics, 30.03.2020 21:46






Mathematics, 30.03.2020 21:46



English, 30.03.2020 21:46

Mathematics, 30.03.2020 21:46

Mathematics, 30.03.2020 21:46


English, 30.03.2020 21:46

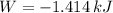 , that means, work is done on the gas, not viceversa.
, that means, work is done on the gas, not viceversa.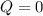 because it is an adiabatic process.
because it is an adiabatic process.
 per atom
per atom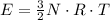 , This result can be experimentally verified or derived from statistical mechanics.
, This result can be experimentally verified or derived from statistical mechanics.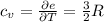

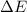 as follows:
as follows: which follows from our first equation.
which follows from our first equation.