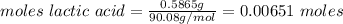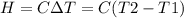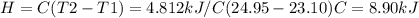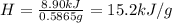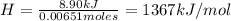Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3
You know the right answer?
A0.5865-g sample of lactic acid (hc3h5o3) is burned in a calorimeter whose heat capacity is 4.812 kj...
Questions

Social Studies, 19.10.2020 07:01


English, 19.10.2020 07:01


Social Studies, 19.10.2020 07:01



Mathematics, 19.10.2020 07:01

Biology, 19.10.2020 07:01

Mathematics, 19.10.2020 07:01


Biology, 19.10.2020 07:01



Mathematics, 19.10.2020 07:01

Physics, 19.10.2020 07:01

Mathematics, 19.10.2020 07:01



History, 19.10.2020 07:01