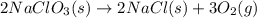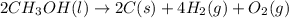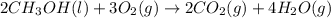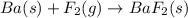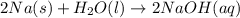Chemistry, 05.09.2019 17:20 skatingby8910
Classify each of the following reactions: i. decopositionii. combinationiii. combustion a. 2ch3oh(l)+3o2(g)→2co2(g)+4h2o(g) b. 2naclo3(s)→2nacl(s)+3o2(g) c. ba(s)+f2(g)→baf2(s) d. 2na(s)+h2o(l)→2naoh(aq) e. 2ch3oh(l)→2c(s)+4h2(g)+o2(g)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:40
Who is better, messi or cristiano, i need this for a chemistry class. asap
Answers: 1

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1
You know the right answer?
Classify each of the following reactions: i. decopositionii. combinationiii. combustion a. 2ch3oh(l)...
Questions




Mathematics, 12.02.2020 12:46

English, 12.02.2020 12:49

Mathematics, 12.02.2020 12:51


History, 12.02.2020 12:53

Chemistry, 12.02.2020 13:05

Mathematics, 12.02.2020 13:06

Computers and Technology, 12.02.2020 13:07

Biology, 12.02.2020 13:08



Mathematics, 12.02.2020 13:09

Mathematics, 12.02.2020 13:13


Mathematics, 12.02.2020 13:28

Social Studies, 12.02.2020 13:28

Mathematics, 12.02.2020 13:28