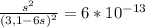Chemistry, 05.09.2019 17:10 wheeler2455
Nickel (ii) ions form a complex ion in the presence of ammonia with a formation constant (kf) of 2.0×10^8:
ni2+ + 6nh3 ⇌ [ni(nh3)6]2+
calculate the molar solubility of nis in 3.1 m nh3. g

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 09:00
Which explanation is true about what happens to a ray of light when it strikes a mirror? a. a light ray is transmitted toward a mirror at a certain angle. the light ray is then reflected by the mirror at an equal angle but in the opposite direction of the transmitted ray. b. an incident ray strikes a mirror at an angle with a line perpendicular to the mirror. the light ray is then reflected at an angle equal to the angle of incidence but on the opposite side of the perpendicular line. c. a reflecting ray strikes a mirror at an angle with a line perpendicular to the mirror. the light ray is then refracted at an angle equal to the angle of the reflecting ray and on the same side of the perpendicular line. d. an incident ray strikes a mirror at an angle with a line parallel to the mirror. the light ray is then transmitted at an angle equal to the angle of incidence but on the opposite side of the parallel line. you so much! : -d take the time to try and answer correctly.
Answers: 3

You know the right answer?
Nickel (ii) ions form a complex ion in the presence of ammonia with a formation constant (kf) of 2.0...
Questions


Mathematics, 04.07.2019 19:00

Mathematics, 04.07.2019 19:00

Social Studies, 04.07.2019 19:00



Mathematics, 04.07.2019 19:00



Mathematics, 04.07.2019 19:00

Mathematics, 04.07.2019 19:00

Mathematics, 04.07.2019 19:00

Computers and Technology, 04.07.2019 19:00

Advanced Placement (AP), 04.07.2019 19:00


Advanced Placement (AP), 04.07.2019 19:00




Social Studies, 04.07.2019 19:00

![\frac{[S^{2-}][Ni(NH3)6^{+2}]}{[NH3]^{6}}](/tpl/images/0223/5248/f04d5.png)