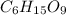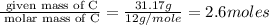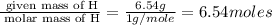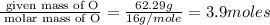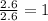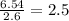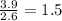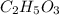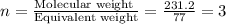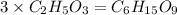Consider a compound that is 31.17% c, 6.54% h, and 62.29% o by mass. assume that we have a 100 g sample of this compound. also consider that the molecular formula mass of this compound is 231.2 amu. what are the subscripts in the actual molecular formula for this compound?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
The overall chemical reaction for photosynthesis isshown below: 6co2 + 6h20 → c6h12o6 + 602what mass of glucose (c6h1206) can form from71.89 g co2? (molar mass of c6h1206 = 180.18g/mol; molar mass of co2 = 44.01 g/mol)71.89 g co2=g c6h1206
Answers: 1

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1
You know the right answer?
Consider a compound that is 31.17% c, 6.54% h, and 62.29% o by mass. assume that we have a 100 g sam...
Questions


Mathematics, 13.07.2020 21:01


Mathematics, 13.07.2020 21:01


Mathematics, 13.07.2020 21:01


Mathematics, 13.07.2020 21:01

Mathematics, 13.07.2020 21:01



Mathematics, 13.07.2020 21:01

Mathematics, 13.07.2020 21:01


Mathematics, 13.07.2020 21:01





Mathematics, 13.07.2020 21:01