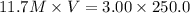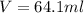Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
You know the right answer?
How do you prepare 250.00 ml of 3.00 m hcl solution starting with a concentrated hcl solution which...
Questions


Mathematics, 05.03.2021 21:40


Mathematics, 05.03.2021 21:40



English, 05.03.2021 21:40


Mathematics, 05.03.2021 21:40

History, 05.03.2021 21:40

Mathematics, 05.03.2021 21:40


Biology, 05.03.2021 21:40

History, 05.03.2021 21:40

Mathematics, 05.03.2021 21:40


Mathematics, 05.03.2021 21:40



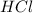 is taken and (250-64.4)= 185.6 ml f water is added to make 250.00 mL of 3.00 M HCl .
is taken and (250-64.4)= 185.6 ml f water is added to make 250.00 mL of 3.00 M HCl .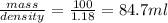
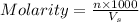
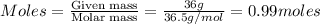
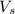 = volume of solution
= volume of solution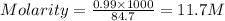
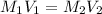
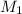 = molarity of stock
= molarity of stock 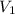 = volume of stock
= volume of stock 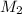 = molarity of diluted
= molarity of diluted 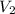 = volume of diluted
= volume of diluted