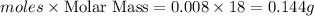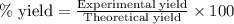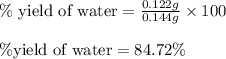Chemistry, 05.09.2019 16:20 kaymillsaps
Gaseous methane (ch4) reacts with gaseous oxygen gas (o2) to produce gaseous carbon dioxide (co2) and gaseous water (h2o) . if 0.122g of water is produced from the reaction of 0.16g of methane and 0.25g of oxygen gas, calculate the percent yield of water.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
Gaseous methane (ch4) reacts with gaseous oxygen gas (o2) to produce gaseous carbon dioxide (co2) an...
Questions


Mathematics, 30.06.2019 05:00

Geography, 30.06.2019 05:00

Biology, 30.06.2019 05:00


Mathematics, 30.06.2019 05:00

Mathematics, 30.06.2019 05:00

Mathematics, 30.06.2019 05:00


Mathematics, 30.06.2019 05:00

Advanced Placement (AP), 30.06.2019 05:00

Mathematics, 30.06.2019 05:00

Mathematics, 30.06.2019 05:00

English, 30.06.2019 05:00


History, 30.06.2019 05:00


History, 30.06.2019 05:00


Mathematics, 30.06.2019 05:00

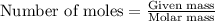 .....(1)
.....(1)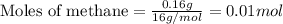
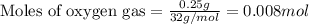
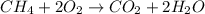
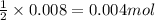 of methane
of methane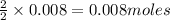 of water
of water