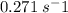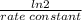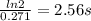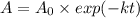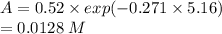Chemistry, 05.09.2019 04:10 mollykay2001p3qo0j
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1.
(a) what is the half-life for this reaction?
(b) if you start with 0.052 m i2 at this temperature, how much will remain after 5.16 s assuming that the iodine atoms do not recombine to form i2?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 23.06.2019 11:00
What are the other two pieces of glassware you used in this experiment that you could obtain hundredths digit accuracy?
Answers: 2

Chemistry, 23.06.2019 11:20
Ajar is tightly sealed at 22°c and 772 torr what is the pressure inside a jar after its been heated to 178°c
Answers: 1
You know the right answer?
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of...
Questions


English, 12.11.2020 07:40

History, 12.11.2020 07:40

Mathematics, 12.11.2020 07:40



Mathematics, 12.11.2020 07:40


Business, 12.11.2020 07:40

History, 12.11.2020 07:40

Mathematics, 12.11.2020 07:40

Biology, 12.11.2020 07:40

Biology, 12.11.2020 07:40

Mathematics, 12.11.2020 07:40

Arts, 12.11.2020 07:40

Biology, 12.11.2020 07:40



English, 12.11.2020 07:40

English, 12.11.2020 07:40