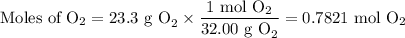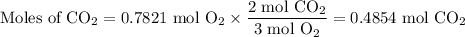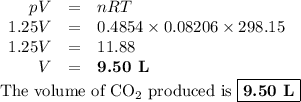Chemistry, 04.09.2019 22:20 bionicboy03120440
The combustion of ethanol (c2h5oh) takes place by the following reaction equation.
c2h5oh (l) + 3 o2 (g) → 2 co2 (g) + 3 h2o (g)
what is the volume of co2 gas produced by the combustion of excess ethanol by 23.3 grams of o2 gas at 25°c and 1.25 atm?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1
You know the right answer?
The combustion of ethanol (c2h5oh) takes place by the following reaction equation.
c2h5oh (l)...
c2h5oh (l)...
Questions

Health, 27.08.2020 14:01

Chemistry, 27.08.2020 14:01


Mathematics, 27.08.2020 14:01


Mathematics, 27.08.2020 14:01

Mathematics, 27.08.2020 14:01

Mathematics, 27.08.2020 14:01

Mathematics, 27.08.2020 14:01

Biology, 27.08.2020 14:01

English, 27.08.2020 14:01

Mathematics, 27.08.2020 14:01

English, 27.08.2020 14:01

English, 27.08.2020 14:01

Mathematics, 27.08.2020 14:01

Computers and Technology, 27.08.2020 14:01

Business, 27.08.2020 14:01

Health, 27.08.2020 14:01

Mathematics, 27.08.2020 14:01

Mathematics, 27.08.2020 14:01