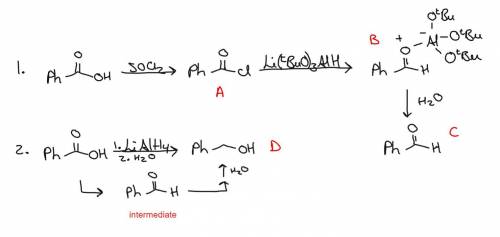
Two students are given the starting material benzoic acid and are asked to prepare benzaldehyde. the first student starts by refluxing her sample of benzoic acid in thionyl chloride in the fume hood. upon completion of the reaction, she evaporates the thionyl chloride to isolate compound a. she treats compound a with a stoichiometric amount of lithium tri-tert-butoxyaluminum hydride at –78 °c in diethyl ether, producing compound b. adding water, she isolates her product, compound c. the second student takes a different route. she treats benzoic acid with an excess of lithium aluminum hydride (lah) in diethyl ether, followed by careful addition of ethyl acetate to remove any unreacted lah. she adds water and isolates her product, compound d. draw the structure for compound a, compound c, and compound d below.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
On a distance vs time graph the line of an object at rest is a
Answers: 1

Chemistry, 21.06.2019 19:30
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3
You know the right answer?
Two students are given the starting material benzoic acid and are asked to prepare benzaldehyde. the...
Questions

Physics, 22.09.2019 07:30

History, 22.09.2019 07:30


Mathematics, 22.09.2019 07:30

World Languages, 22.09.2019 07:30

English, 22.09.2019 07:30


Chemistry, 22.09.2019 07:30


English, 22.09.2019 07:30





History, 22.09.2019 07:30


English, 22.09.2019 07:30

Mathematics, 22.09.2019 07:30


English, 22.09.2019 07:30