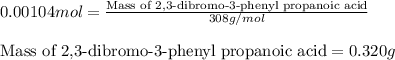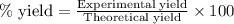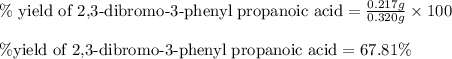Suppose a student started with 155.0 mg of trans-cinnamic acid, 458 mg of pyridinium tribromide, and 2.35 ml of glacial acetic acid. after the reaction and workup, the student ended up with 0.2170 g of brominated product. calculate the student\'s theoretical and percent yields.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2



Chemistry, 23.06.2019 07:20
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)
Answers: 1
You know the right answer?
Suppose a student started with 155.0 mg of trans-cinnamic acid, 458 mg of pyridinium tribromide, and...
Questions




History, 21.09.2021 18:00

Biology, 21.09.2021 18:00

History, 21.09.2021 18:00


Advanced Placement (AP), 21.09.2021 18:00

Computers and Technology, 21.09.2021 18:00


English, 21.09.2021 18:00


History, 21.09.2021 18:00


Mathematics, 21.09.2021 18:00

History, 21.09.2021 18:00


English, 21.09.2021 18:00

Mathematics, 21.09.2021 18:10

Business, 21.09.2021 18:10

 .....(1)
.....(1)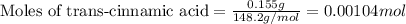
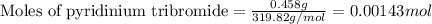
![\text{trans-cinnamic acid}+\text{pyridinium tribromide}\xrightarrow[]{CH_3COOH}\text{2,3-dibromo-3-phenyl propanoic acid}](/tpl/images/0222/4310/95be0.png)
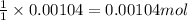 of pyridinium tribromide
of pyridinium tribromide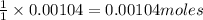 of 2,3-dibromo-3-phenyl propanoic acid
of 2,3-dibromo-3-phenyl propanoic acid