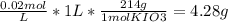Chemistry, 04.09.2019 03:20 JosefineRubino2204
You wish to prepare 1 l of a 0.02 m potassium iodate solution. you require that the final concentration be within 1% of 0.02 m and that the concentration must be known accurately to the fourth decimal place. how would you prepare this solution? specify the glassware you would use, the accuracy needed for the balance, and the ranges of acceptable masses of kio3 that could be used.(a) to make this solution (ideally) you would need grams of potassium iodide dissolved in enough water to make up 1 l of solution. fill in the blank(b)what is the least accurate balance that could be used to make this solution?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3
You know the right answer?
You wish to prepare 1 l of a 0.02 m potassium iodate solution. you require that the final concentrat...
Questions

Chemistry, 29.08.2019 04:00


Health, 29.08.2019 04:00

History, 29.08.2019 04:00

Social Studies, 29.08.2019 04:00

Chemistry, 29.08.2019 04:00

Business, 29.08.2019 04:00







English, 29.08.2019 04:00

Social Studies, 29.08.2019 04:00



Computers and Technology, 29.08.2019 04:00