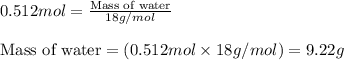Chemistry, 04.09.2019 02:30 dzepeda061
2n2h4(g)+ n2o4(g) à3 n2(g) + 4 h2o(g) when 8.0 g of n2h4(32 g mol-1) and 18.4 g of n2o4(92 g mol-1) are mixed together and react according to the equation above, what is the maximum mass of h2o that can be produced?

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 23.06.2019 06:00
Nthis lab, you will do experiments to identify types of changes. using the question format you learned (shown above), write an investigative question that you can answer by doing these experiments
Answers: 3
You know the right answer?
2n2h4(g)+ n2o4(g) à3 n2(g) + 4 h2o(g) when 8.0 g of n2h4(32 g mol-1) and 18.4 g of n2o4(92 g mol-1)...
Questions


Mathematics, 27.08.2019 15:30


Mathematics, 27.08.2019 15:30


Geography, 27.08.2019 15:30


History, 27.08.2019 15:30

History, 27.08.2019 15:30




Arts, 27.08.2019 15:30

English, 27.08.2019 15:30






Biology, 27.08.2019 15:30

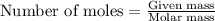 .....(1)
.....(1)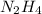 :
: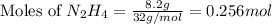
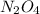 :
: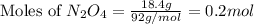
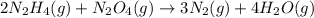
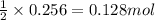 of
of 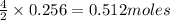 of water
of water