
Chemistry, 04.09.2019 02:20 jeanetteelliotp9ru6m
Consider two gases, a and b, each in a 1.0-l container with both gases at the same temperature and pressure. the mass of gas a in the container is 0.34 g and the mass of gas b in the container is 0.48 g.
a. which gas sample has the most molecules present? b. which gas sample has the largest average kinetic energy? c. which gas sample has the fastest average velocity? d. how can the pressure in the 2 containers be equal to each other since the larger gas b molecules collide with the container walls more forcefully?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What type of reaction is represented by the following example? 2co2 (g) + 4h2o (l) + 1452 kj 2ch3oh (l) (g) + 3o2 (g) exothermic endothermic
Answers: 1



Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
You know the right answer?
Consider two gases, a and b, each in a 1.0-l container with both gases at the same temperature and p...
Questions






Social Studies, 19.08.2019 04:30

Social Studies, 19.08.2019 04:30





Mathematics, 19.08.2019 04:30



Health, 19.08.2019 04:30

Social Studies, 19.08.2019 04:30



Physics, 19.08.2019 04:30

Mathematics, 19.08.2019 04:50

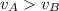 D) compensation between force and velocity.
D) compensation between force and velocity.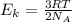 , where R is the ideal gas constant, T is the temperature and
, where R is the ideal gas constant, T is the temperature and 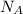 is the Avogadro´s number. All this values are the same in both gases thus they have the same kinetic energy.
is the Avogadro´s number. All this values are the same in both gases thus they have the same kinetic energy.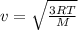 , where M is the molecular mass, if we want to know wich gas has fastest velocity is enough to know the rate between two velocities.
, where M is the molecular mass, if we want to know wich gas has fastest velocity is enough to know the rate between two velocities.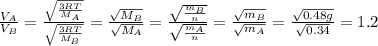 , due to rate is greater than 1
, due to rate is greater than 1 

