Iron fluoride (fef2) dissociates according to the following equation:
fef2(s) ⇌ fe^2+(aq) + 2...

Chemistry, 04.09.2019 01:10 SassyChumpkins
Iron fluoride (fef2) dissociates according to the following equation:
fef2(s) ⇌ fe^2+(aq) + 2 f^–(aq)
calculate the solubility of fef2 in water by using 2.36 x 10^-6 as the solubility product.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which one of the following gases is not an important component of soil?
Answers: 2

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3
You know the right answer?
Questions


Mathematics, 17.02.2021 14:50

Biology, 17.02.2021 14:50

World Languages, 17.02.2021 14:50

English, 17.02.2021 14:50

English, 17.02.2021 14:50


Biology, 17.02.2021 14:50

Mathematics, 17.02.2021 14:50


Computers and Technology, 17.02.2021 14:50



Mathematics, 17.02.2021 14:50



Mathematics, 17.02.2021 14:50

Mathematics, 17.02.2021 14:50


![Kps = \frac{[product]^x}{[reagent]^y}](/tpl/images/0222/2627/6a1f3.png)
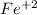 is necessary 2 mols of
is necessary 2 mols of 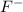 , so if we call "x" the molar concentration of
, so if we call "x" the molar concentration of 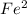 , for
, for ![Kps = [Fe^{+2}].[F^-]^2\\\\2.36x10^{-6} = x(2x)^2\\\\2.36x10^{-6} = 4x^3\\\\x^3 = 5.9x10^{-7}\\\\x = \sqrt[3]{5.9x10^{-7}} \\\\x = 8.4x10^{-3} mol/L](/tpl/images/0222/2627/99690.png)


