
Chemistry, 04.09.2019 00:30 wyattjefferds05
Trevor dissolves sodium hydroxide pellets in a beaker of water at room temperature, and notes that the beaker becomes warm. which correctly designates the signs of δh, δs, and δg for this process?
a. δh > 0, δs > 0, and δg < 0
b. δh < 0, δs > 0, and δg < 0
c. δh > 0, δs > 0, and δg > 0
d. δh < 0, δs < 0, and δg > 0

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1
You know the right answer?
Trevor dissolves sodium hydroxide pellets in a beaker of water at room temperature, and notes that t...
Questions

Business, 10.10.2019 07:30




Spanish, 10.10.2019 07:30


History, 10.10.2019 07:30



Biology, 10.10.2019 07:30



History, 10.10.2019 07:30

Mathematics, 10.10.2019 07:30


Mathematics, 10.10.2019 07:30


Biology, 10.10.2019 07:30


Mathematics, 10.10.2019 07:30

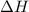 for Exothermic reaction is negative and
for Exothermic reaction is negative and 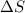 is positive when randomness increases and
is positive when randomness increases and 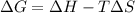
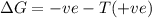
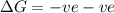
 will be negative.
will be negative.

