Chemistry, 04.09.2019 00:10 ashley5196
Sulfuric acid (h2so4) is prepared commercially from elemental sulfur using the contact process. in a typical sequence of reactions, the sulfur is first burned: s + o2 → so2 , then it is converted to so3 using a catalyst: 2 so2 + o2 → 2 so3 . the resulting so3 is reacted with water to produce the desired product: so3 + h2o → h2so4 . how much sulfuric acid could be prepared from 83 moles of sulfur? answer in units of g.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1

Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1

Chemistry, 23.06.2019 15:00
How many more valence electrons does sodium need to have a full outer valence shell
Answers: 3
You know the right answer?
Sulfuric acid (h2so4) is prepared commercially from elemental sulfur using the contact process. in a...
Questions


Business, 11.10.2020 20:01

English, 11.10.2020 20:01


Mathematics, 11.10.2020 20:01

Mathematics, 11.10.2020 20:01

Engineering, 11.10.2020 20:01


Computers and Technology, 11.10.2020 20:01

Chemistry, 11.10.2020 20:01


Mathematics, 11.10.2020 20:01



Arts, 11.10.2020 20:01

Mathematics, 11.10.2020 20:01

Mathematics, 11.10.2020 20:01

Social Studies, 11.10.2020 20:01

Biology, 11.10.2020 20:01

Advanced Placement (AP), 11.10.2020 20:01

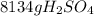
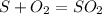
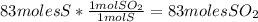
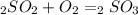
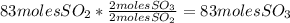
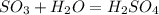
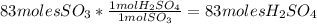
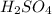 :
: