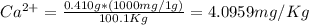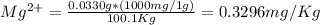Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1


You know the right answer?
Wastewater from a cement factory contains 0.410 g of ca2+ ion and 0.0330 g of mg2+ ion per 100.0 l o...
Questions



Chemistry, 24.09.2019 04:30

Mathematics, 24.09.2019 04:30


History, 24.09.2019 04:30

Physics, 24.09.2019 04:30

History, 24.09.2019 04:30

Chemistry, 24.09.2019 04:30


Physics, 24.09.2019 04:30




Mathematics, 24.09.2019 04:30




Mathematics, 24.09.2019 04:30

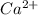 = 4.0959 ppm
= 4.0959 ppm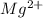 = 0.3296 ppm
= 0.3296 ppm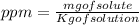
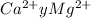
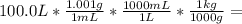 = 100.1 kg
= 100.1 kg