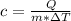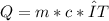Chemistry, 03.09.2019 18:30 hannahsparks7073
The specific heat capacity of a pure substance can be found by dividing the heat needed to change the temperature of a sample of the substance by the mass of the sample and by the change in temperature. the heat capacity of a certain substance has been measured to be2.76j·g°c suppose 466.g of the substance are heated until the temperature of the sample has changed by 39.4°c. write an equation that will let you calculate the heatqthat was needed for this temperature change. your equation should contain only symbols. be sure you define each symbol.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2
You know the right answer?
The specific heat capacity of a pure substance can be found by dividing the heat needed to change th...
Questions