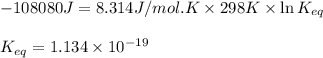Chemistry, 03.09.2019 05:10 kjmccarty02
Determine the direction in which the following reaction is spontaneous at 25oc: mg2+(aq) + k(s) < > mg(s) + k+(aq) determine the equilibrium constant k and go using the cell potential for this reaction and which will be the anode and cathode?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1
You know the right answer?
Determine the direction in which the following reaction is spontaneous at 25oc: mg2+(aq) + k(s) <...
Questions

Mathematics, 28.04.2021 15:10



English, 28.04.2021 15:10

French, 28.04.2021 15:10

Chemistry, 28.04.2021 15:10

Mathematics, 28.04.2021 15:10


Mathematics, 28.04.2021 15:10

Mathematics, 28.04.2021 15:10

Mathematics, 28.04.2021 15:10






Mathematics, 28.04.2021 15:10



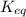 and
and 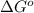 of the reaction is
of the reaction is 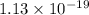 and -108080 J respectively.
and -108080 J respectively. 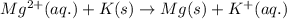
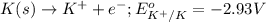
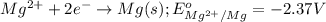
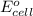 of the reaction, we use the equation:
of the reaction, we use the equation: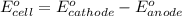
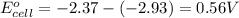
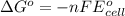
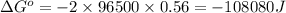
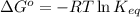
![25^oC=[273+25]=298K](/tpl/images/0221/6855/6a9f9.png)