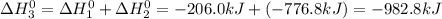Calculate ho298 for the process zn + s + 2o2 ? znso4 from the following information:
zn + s...


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2


Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
You know the right answer?
Questions

Mathematics, 02.03.2021 14:00


Mathematics, 02.03.2021 14:00

Mathematics, 02.03.2021 14:00



Physics, 02.03.2021 14:00

Geography, 02.03.2021 14:00

Mathematics, 02.03.2021 14:00



English, 02.03.2021 14:00

Mathematics, 02.03.2021 14:00



Mathematics, 02.03.2021 14:00


Mathematics, 02.03.2021 14:00

History, 02.03.2021 14:00

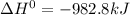 .
.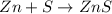
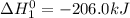 (1)
(1)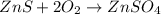
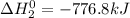 (2)
(2)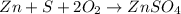
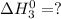 (3)
(3)