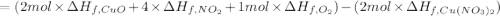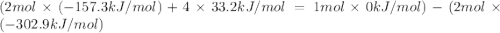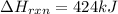The decomposition of copper(ii) nitrate on heating is endothermic reaction. 2cu(no3)2(s) → 2c10(s) + 4no2(g) + o2(g) calculate the enthalpy change for this reaction using the following enthalpy changes of formation. ah! [cu(no3)2) = -302.9 kj mol? ah, (cuo) = -157.3 kj mol? . ah[no2) = +33.2 kj mol.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2
You know the right answer?
The decomposition of copper(ii) nitrate on heating is endothermic reaction. 2cu(no3)2(s) → 2c10(s) +...
Questions


Biology, 15.10.2019 06:00

Mathematics, 15.10.2019 06:10

History, 15.10.2019 06:10


Mathematics, 15.10.2019 06:10




History, 15.10.2019 06:10



History, 15.10.2019 06:10

Mathematics, 15.10.2019 06:10

Mathematics, 15.10.2019 06:10


Mathematics, 15.10.2019 06:10



Biology, 15.10.2019 06:10

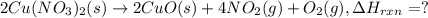
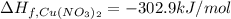
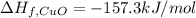
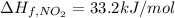
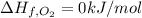 (standard state)
(standard state)![\Delta H_{rxn}=\sum [\Delta H_f(product)]-\sum [\Delta H_f(reactant)]](/tpl/images/0221/5790/84aad.png)
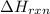 =
=